SITUATION
In recent months, several European countries have experienced a surge in cases of pertussis, commonly known as whooping cough. This highly contagious respiratory illness spreads through droplet infection, posing significant risks, particularly to unvaccinated young children and the elderly. The increase in cases appears to be affecting all age groups, with the majority of cases being diagnosed among children 15-19 years of age and among vaccinated individuals.
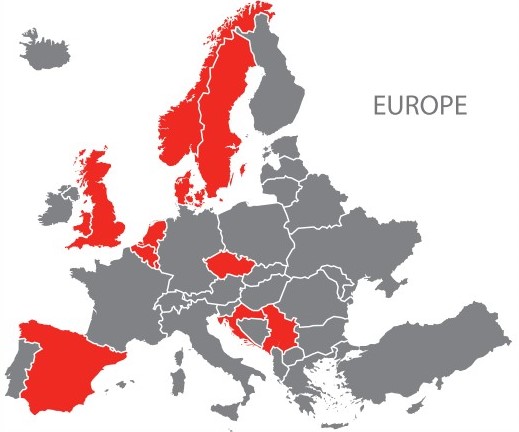
Countries in Europe affected by the outbreak include Belgium, Croatia, Czechia, Denmark, Luxembourg, Serbia, Spain, Sweden, the United Kingdom, the Netherlands, and Norway. Countries outside of Europe are also affected, including Australia, Brazil, Bolivia, Canada, Israel, and the United States of America.1
GENEPROOF RESPONSE
It is essential to prioritize swift and accurate diagnostic testing to minimize the impact of the pertussis outbreak. If whooping cough is suspected, timely detection of the causative bacterium is critical. The GeneProof Bordetella pertussis/parapertussis PCR kit offers a reliable solution for this purpose. Compared to conventional culture methods, PCR provides faster results, enabling prompt initiation of antibiotic treatment. By identifying Bordetella pertussis early, we can effectively contain the spread of the disease and protect public health. Health networks worldwide are encouraged to suspect and test for pertussis and initiate treatment early among cases and contacts, in accordance with national guidelines and recommendations.
The GeneProof Bordetella pertussis/parapertussis PCR kit offers a reliable solution for this objective. Compared to conventional culture methods, PCR provides faster results, enabling the prompt initiation of antibiotic treatment. Early identification of Bordetella pertussis is crucial for effectively containing the spread of the disease and protecting public health.
HIGHLY SPECIFIC DETECTION
Amplification of the multi-copy insertion sequences IS1002 (specific for both Bordetella pertussis/parapertussis) and IS1001 (specific only for B parapertussis). No falsepositive results for Bordetella holmesii.
ACCURACY
Analytical Sensitivity: (LoD with 95% probability) 0.212 cp/µl Analytical Specificity: Bordetella pertussis (BP) 100 %, Bordetella parapertussis (BPP) 100 %
| PCR Kit |
25 Reaction Kits |
100 Reaction Kits |
|
GeneProof Bordetella pertussis/parapertussis PCR Kit
|
BP/ISEX/025 |
BP/ISEX/100 |
Source: 1. https://www.ecdc.europa.eu/sites/default/files/documents/communicable-disease-threats-report-week-12-2024.pdf
Trust GeneProof for targeted molecular diagnostic solutions. Learn more.
Download from here for more information